Warm-Up Exercise 8
Due 12:15 pm, Fri 14 Sep 2012
Physics 123, Fall 2012
Reading assignment: 20.4-20.6
What is a "state variable"? In your own words, and without referring to the text if possible, why
do things like temperature, internal energy, volume, and pressure fall into this
category?
A state variable is something that helps specify the state of the entire system. They describe macroscopic quantities. State variables are often part of an "equation of state" that describes the dependence of the system's state on these variables. The given quantities fall into this category because individual molecules contribute to temperature, pressure, etc., but T and P measure the contributions from ALL molecules.
Enter "-", "+", or "0" for the quantities Q, W, and ΔEint (three entries on each line) for the following situations (this is Quick Quiz 20.3 in the Eighth edition of the text).
(Note: newer editions of the textbook talk about W as the work done on
the gas; older editions may describe W as the work done by the gas,
which is the negative of the other W. I will try to always follow the newer
convention.)
(A) Rapidly pumping up a bicycle tire (the system in question is the
air in the pump)
(B) Lukewarm water in a pan on a hot stove (the system in question is
the water in the pan)
(C) Air quickly leaking out of a balloon (the system in question is
the air that was originally in the balloon)
Q W deltaE
(A) 0 + +
(B) + 0 +
(C) 0 - -
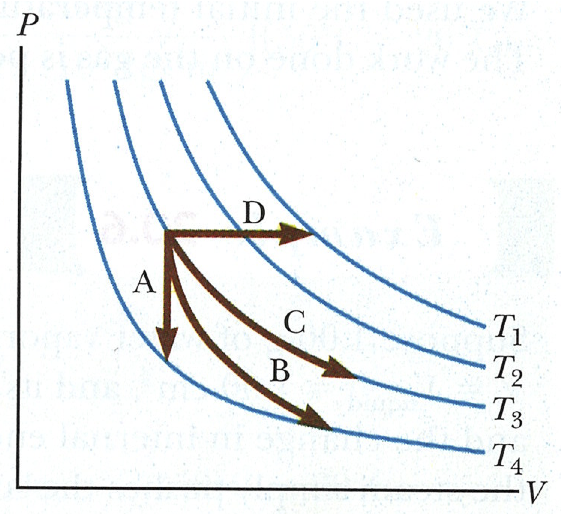
Regarding the P-V diagram above, match the letters A-D to the appropriate path: isobaric, isovolumetric,
isothermal, or adiabatic. Which processes do you think are more common in typical
situations (motors, heaters, calorimeters, refrigerators,
leaf blowers, etc.)?
A - isovolumetric (fixed volume).
B - adiabatic
C - isothermic (temperature is not changing)
D - isobaric (pressure not changing. "iso" means "the same", "baric" refers to pressure.)
In many applications (motors) adiabatic and isovolumetric processes are common (see Otto process in Chap. 22).
In many science experiments, isothermal processes are common.
Isobaric processes are important when the system is open to the atmosphere, such as boiling water on the stove.
I guess they are all important! :-)
Return to Course Page
