Warm-Up Exercise 10
Due 12:15 pm, Wed 19 Sep 2012
Physics 123, Fall 2012
Reading assignment: 22.1,22.5
Give your own example of some process or event where the first law of
thermodynamics (what is that again?) is not violated, but the event
will not happen because it violates the second law of thermodynamics.
Here are two:
* All of the hot molecules in the air in my room suddenly congregate right near my body and cause me to spontaneously combust.
* A ball on the floor suddenly gathers kinetic energy from the surrounding molecules in the floor. This causes the floor to cool down a small amount and the ball leaps into the air.
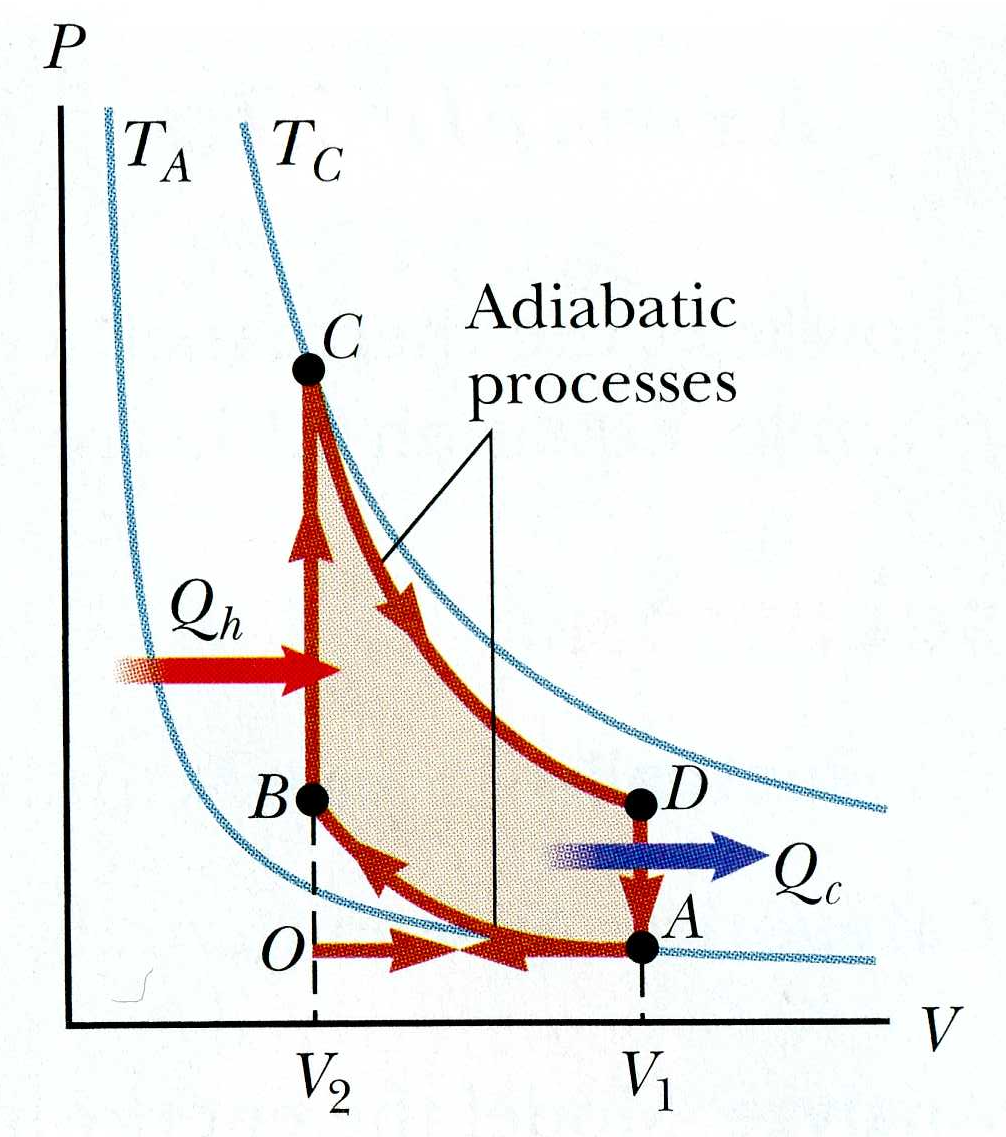
In the book's discussion of the gasoline engine, work is done
on the gas during step 2 of the
cyclic process (the "compression stroke", going from A to B). How can it work as an engine if we are doing work on
it instead of getting work out?! Explain using your own words and
using the P-V diagram.
The gas in any cyclical engine must have work done *on it* by its surroundings during part of the cycle as the path goes right-to-left in the P-V diagram. But it also does work *on the surroundings* as the path goes left-to-right. If the cycle is clockwise, then there is more area (and more work) in the latter than in the former. We can then extract some net work from the engine. The source of that energy is heat: there is a net positive Q added to the gas during the cycle.
Return to Course Page
