Reading assignment: 22.6-22.7





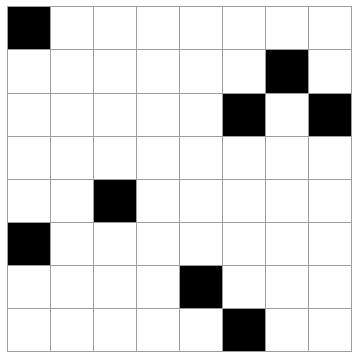
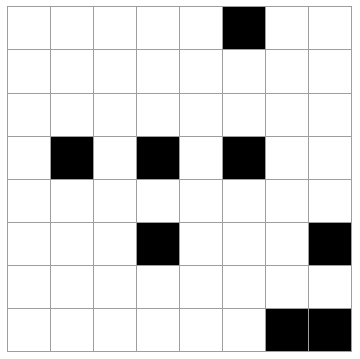
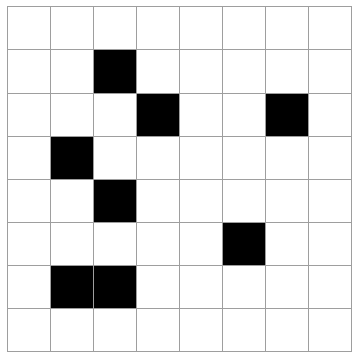
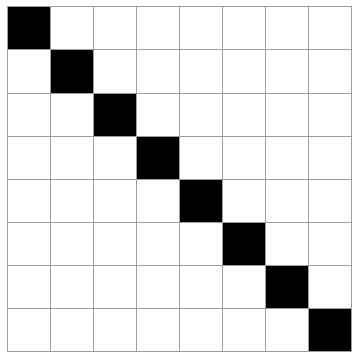
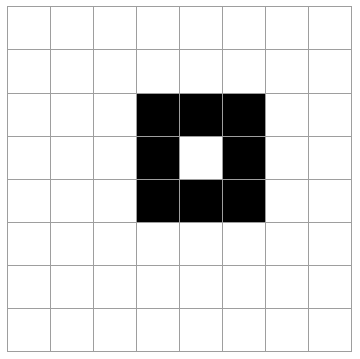
Imagine a system like a checkerboard (8x8 grid) where 8 of the 64 spaces may be occupied, the rest empty. Selecting the 8 occupied squares at random, there are a lot of possible outcomes (how many?).
The 5 cases above are possible outcomes. Rank these in order of least likely to most likely. Explain your reasoning.
All of the states are equally likely. Every microstate is as likely as any other. Of course, this is not true for *macrostates*. (By the way, there are "64 choose 8" = 4,426,165,368 total microstates like this. See here for the formula if you are unfamiliar with it.)
Using the same situation as in the first problem, imagine a *macrostate* that is composed of all of the microstates where all of the occupied squares touch at least one other occupied square (diagonally or adjacent). Compare that to a second macrostate where only 2 or fewer of the 8 occupied squares are touching each other. Which of these macrostates is more likely? Explain.
Without doing any calculations, the second kind of macrostate seems much more likely than the first. On an 8x8 grid, selecting 8 spots at random, it's fairly rare to select 8 spots that are *all* connected. It would much less likely than having two spots touching.
What's the difference between a macrostate and a microstate? How does that difference come into play in your answers to questions 1 and 2?
A macrostate the "big perspective" on a system, described by macroscopic state variables which describe a feature of the *whole* system. A microstate completely specificies all variables in the system. For example, a macrostate could be a gas at a given pressure; the microstate would be a particular set of molecular speeds and directions that give rise to that pressure.
Consider all of the gas particles in the room where you are sitting right now. Thinking about all the positions, speeds, and directions of the particles in the room, is this a likely macrostate? or an unlikely one? Also, describe a macrostate (not microstate), for the gas particles in this room, with the same energy that is quite different from this one.
This is a very likely macrostate, which is in fact the reason why the temperature/pressure/etc. in your room are what they are. A very *unlikely* macrostate would be one where the velocities of all particles are the same, but all of the fast-moving particles are in one half of the room and all of the slow molecules are in the other half.